Our Product
RenoVō® is a liquid, acellular, equine amniotic tissue allograft comprised of amnion and amniotic fluid, intended to supplement and protect tissues.
RenoVō® is cryopreserved and provided frozen in three volumes.
Item Number Size:
RV-010150 1.5 cc
RV-010300 3.0 cc
RV-010500 5.0 cc
Amnion and amniotic fluid are rich sources of biologically active factors involved in tissue regeneration with reported anti-inflammatory, anti-bacterial, re-epithelialization, and anti-fibrotic properties.1, 2 Amniotic tissues contain key growth factors, amino acid, hyaluronic acid, proteoglycans, and extracellular matrix proteins recognized as intrinsic to the complex tissue healing process.

Biological Factors Identified in RenoVō®
RenoVō® is enjoying rapid adoption as a superior alternative to autologous and other therapies. Ready availability of an allograft potentiates clinical use without having time lapse for culturing cells or harvest techniques required for PRP and other autologous therapies. Due to individual variability within products such as PRP, cytokine and growth factor profiles can vary dramatically between patients. The reproducibility of each donor tissue also offers a more homogeneous protein concentration and product consistency in comparison to other regenerative modalities. RenoVō® is cryopreserved and maintained frozen before use, to preserve beneficial proteins that may not survive lyophilization. Clinicians currently managing injuries with PRP or autologous therapy should consider use of RenoVō® to achieve similar or improved clinical outcomes.

Functions & Proteins
Anti-Inflammatory
Anti-Fibrotic
Immunomodulatory
Angiogenic
Biological Factors Identified in RenoVō®
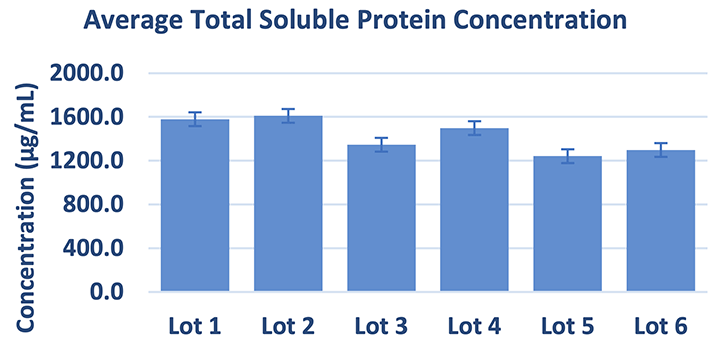
Average total soluble protein concentration measured in RenoVō® (n=24, determined by a third party laboratory)
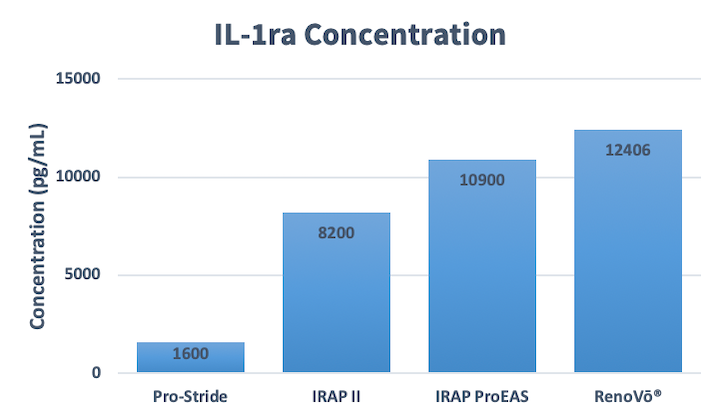
High Levels of Interleukin-1 Receptor Antagonist Protein
When comparing the average IL-1ra concentration to other modalities, the measured average IL-1ra concentration detected in RenoVō® was 12,406 pg/mL (n=24, determined by a third party laboratory) compared to 1,600 pg/mL in Pro-Stride, 8,200 pg/mL in IRAP-II, and 10,900 pg/mL in IRAP ProEAS (Arthrex® VetSystems, vLA1-000004-en-US_A)

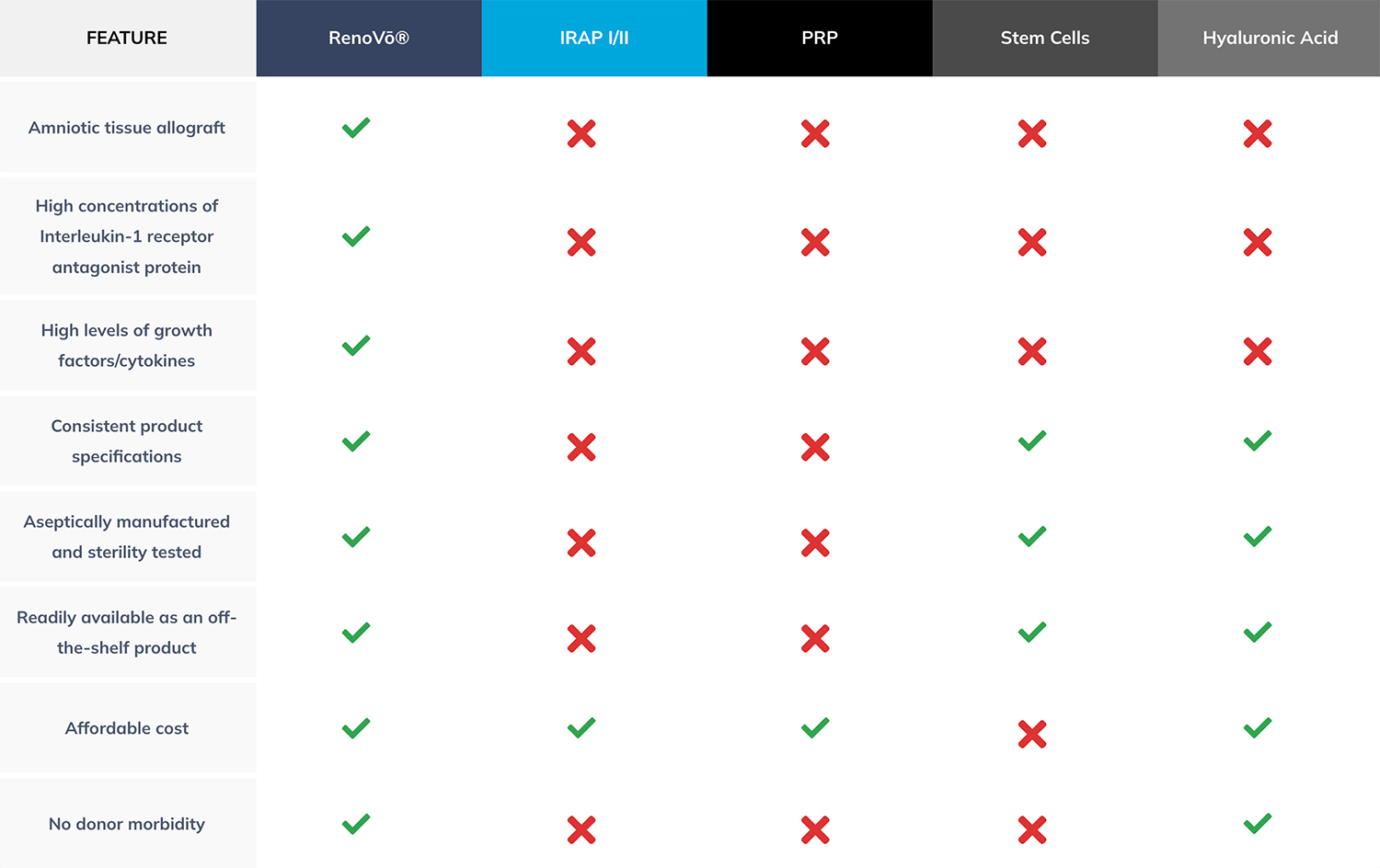
The RenoVō® Advantage
References:
1. Amniotic Fluid: Not Just Fetal Urine Anymore. Mark A Underwood, William M Gilbert, Michael P Sherman. Journal of Perinatology. 2005; 25: 341-348
2. Growth factors and cytokines in wound healing. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Wound Repair Regen. 2008; 16(5): 585-601




